Nh4oh hires stock photography and images Alamy
NH4OH*HNO3 molar mass. Molar mass of NH4OH*HNO3 is 98.0586 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between NH4OH*HNO3 weight and moles. Compound.
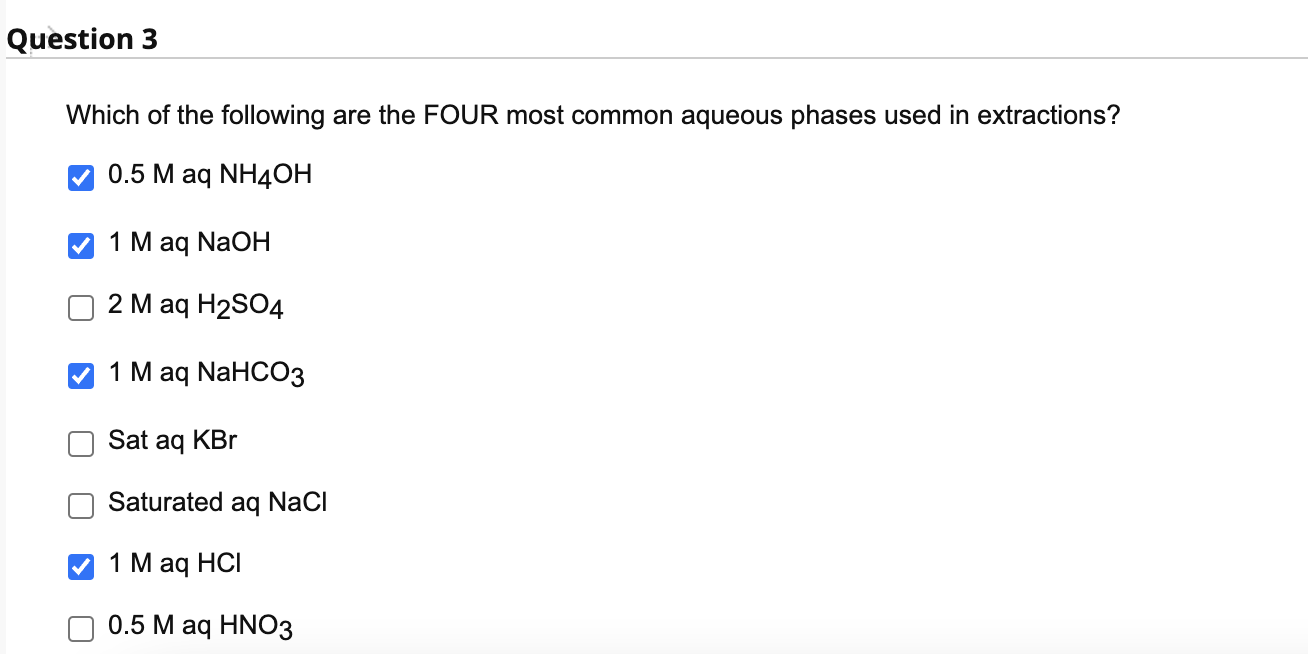
Solved Which of the following are the FOUR most common
There are three main steps for writing the net ionic equation for AgNO3 + NH4OH = AgOH + NH4NO3 (Silver nitrate + Ammonium hydroxide). First, we balance the.

NH4OH + HNO3 → NH4NO3 + H2O Реакция гидрата аммиака и азотной кислоты YouTube
HNO3 | nitric acid | solid + NH4OH | ammonium hydroxide | solid = H2O | water | solid + NH4NO3 | ammonium nitrate | solid | Temperature: temperature, Other Condition excess chlorine Introduce Detailed information about the equation

How to Balance NH4OH + HNO3 = NH4NO3 + H2O (Ammonium hydroxide + Nitric acid) YouTube
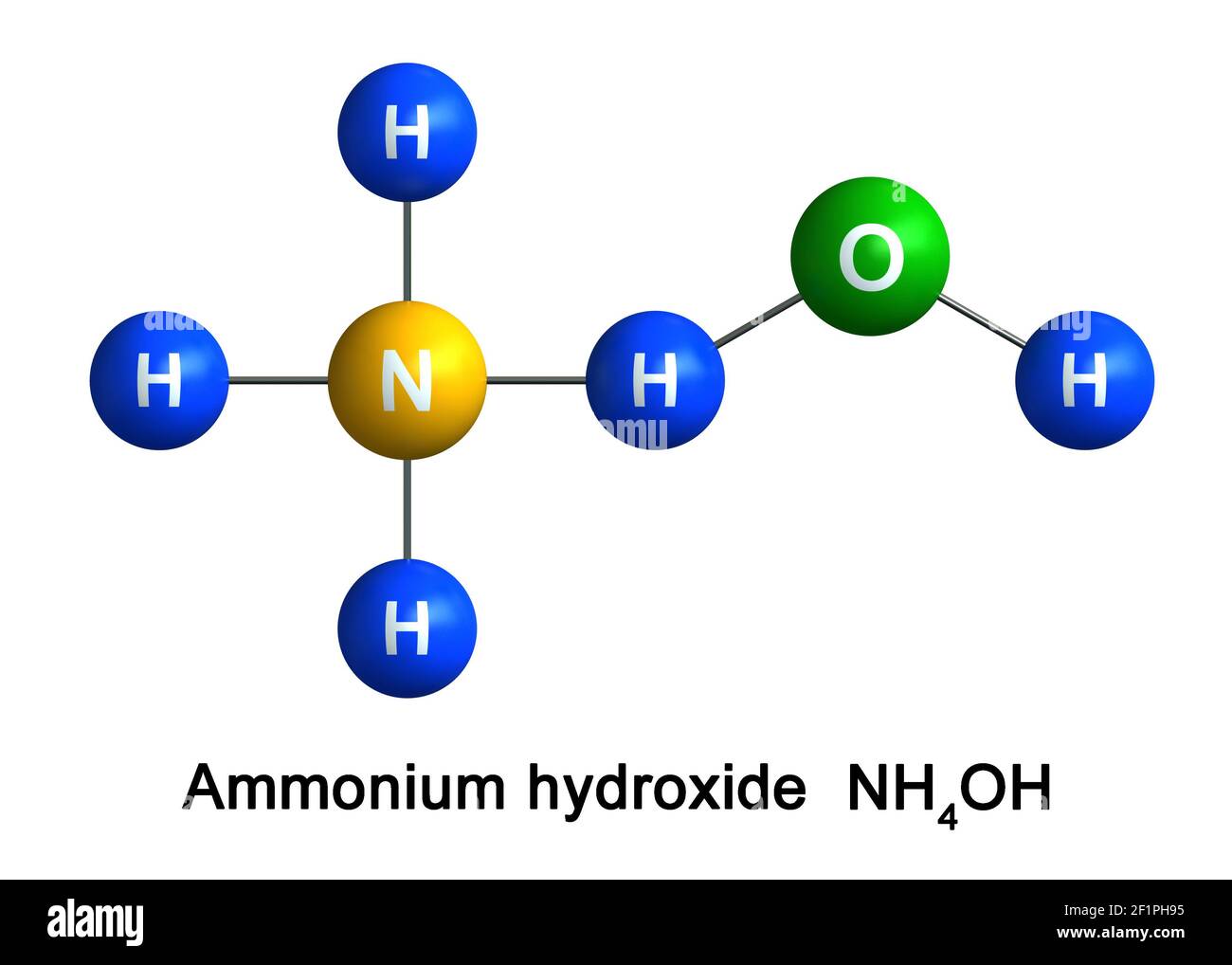
Description. Ammonium hydroxide appears as a colorless aqueous solution. Concentration of ammonia ranges up to approximately 30%. Ammonia vapors (which arise from the solution) irritate the eyes. CAMEO Chemicals. Ammonium hydroxide is a solution of ammonia in water. It has a role as a food acidity regulator. ChEBI.

How to balance NH4OH+HNO3=NH4NO3=+H2OReaction balance NH4OH+HNO3=NH4NO3+H2ONH4OH+HNO3=NH4NO3
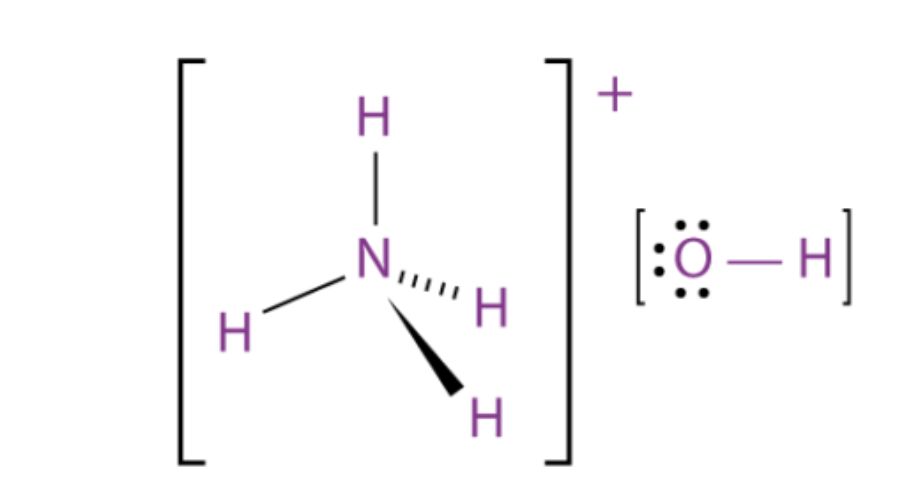
You can either write $\ce{NH4OH}$ more conventionally (or in a more modern way) as ammonia in aqueous solution, $\ce{NH3(aq)}$, which is a weak base. Or you can recognize $\ce{OH-}$ in ammonium hydroxide as a strong base, which would react with the ammonium to make ammonia. In either way of looking at things, the conjugate base is present as well.

Al Oh 3 Hno3 Estudiar
Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The equation is balanced. Balancing with algebraic method. This method uses algebraic equations to find the correct coefficients.

NH4OH là gì? Đặc điểm tính chất ứng dụng Amoni hydroxit
Balance NH4OH + HNO3 = NHNO3 + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the beginning.

NH4OH Lewis Dot Structure (Ammonium Hydroxide) YouTube
Free Chemical Reactions calculator - Calculate chemical reactions step-by-step

Амоніак та його властивості презентація з хімії
HNO3+NH4OH = en. Related Symbolab blog posts. Practice Makes Perfect. Learning math takes practice, lots of practice. Just like running, it takes practice and dedication. If you want. Read More. Enter a problem. Cooking Calculators. Cooking Measurement Converter Cooking Ingredient Converter Cake Pan Converter See more.

The Surprising Reaction Of Nitric Acid & Ammonia The Chemistry Blog
Add a comment. -1. The products will be HNOX3 +NHX4OH H N O X 3 + N H X 4 O H. This is because a salt hydrolysis reaction always produces an acid and a base. The HX+ H X + in HX2O H X 2 O will bond with NOX3X− N O X 3 X − to form HNOX3 H N O X 3, a strong acid. The remaining OHX− O H X − in HX2O H X 2 O will bond with NHX4X+ N H X 4 X.

Ammonium hydroxide, ammonia solution, NH4OH molecule. Skeletal chemical formula. Vector
Balance HNO3 + NH3OH = H2O + NH4NO3 Using the Algebraic Method To balance the equation HNO3 + NH3OH = H2O + NH4NO3 using the algebraic method step-by-step, you must have experience solving systems of linear equations.
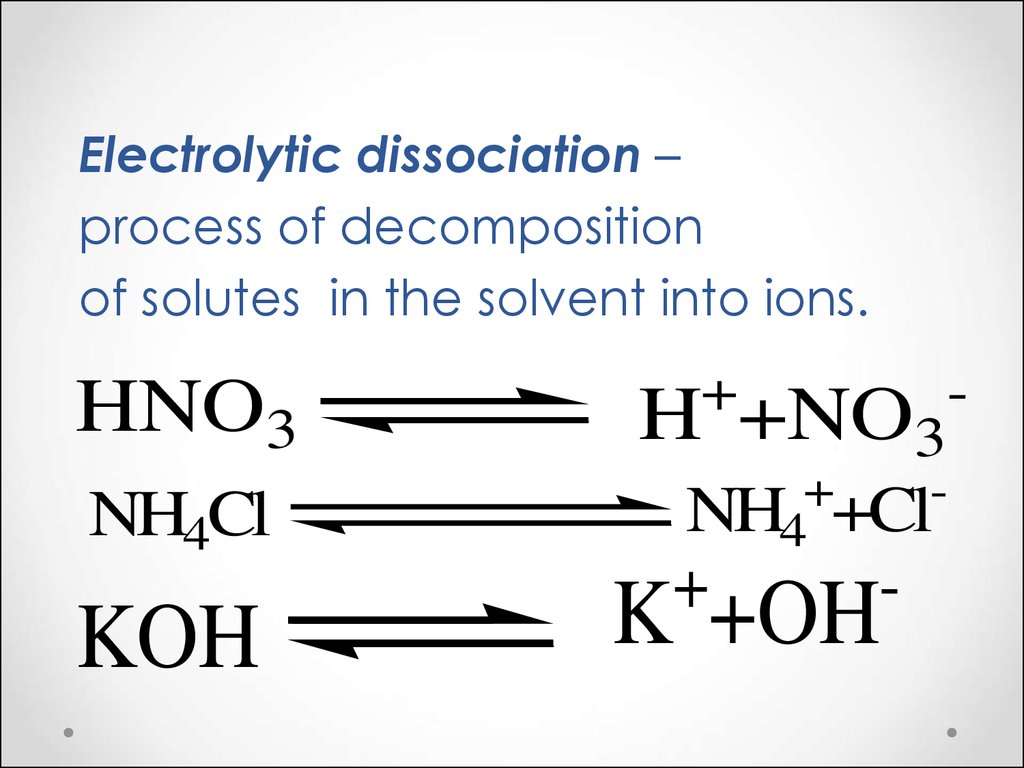
Solutions. Acidbase equilibrium in biological systems презентация онлайн
Check the balance. Now, both sides have 4 H atoms and 2 O atoms. The equation is balanced. Balancing with algebraic method. This method uses algebraic equations to find the correct coefficients.

NH4OH AMMONIUM HYDROXIDE 0935161826
Chemistry. Chemistry questions and answers. Given: NH4NO3 (s) + H2O (l) --> NH4OH (aq) + HNO3 (aq) 50 mL of water Temperature change: -3.8°C Mass of NH4NO3 3.03g a) Calculate the heat associated with water q (H2O) ____J b) the heat either released or absorbed by the reaction (Change in Hrxn) _____J c) Calculate the change in enthalpy (change.

SOLVED The heat of neutralisation is highest for the reaction between A. NH4OH + CH3COOH B
In this video we'll balance the equation NH4OH + HNO3 = NH4NO3 + H2O and provide the correct coefficients for each compound.To balance NH4OH + HNO3 = NH4NO3.
NH4OH Là Gì ? Tính Chất Và Ứng Dụng Quan Trọng Hoá Chất Trần Tiến
Since there is an equal number of each element in the reactants and products of HNO3 + NH4OH = N2O + 3H2O, the equation is balanced. Balance HNO3 + NH4OH = N2O + H2O Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical.

How to Write the Net Ionic Equation for Fe(NO3)3 + NH4OH = NH4NO3 + Fe(OH)3 YouTube
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of NH4OH + HNO3 = NH4NO3 + H2O, the equation is balanced.