
Carbohidratos. Reacción de Molisch. YouTube
Hans Molisch (6 December 1856, Brünn, Habsburg Moravia - 8 December 1937, Wien, Austria) was a Czech-Austrian botanist. Molisch's test is named after him, it is a sensitive chemical test for the presence of carbohydrates.

Hans Molisch (18561937), Plant Anatomy and Physiology 650 plus
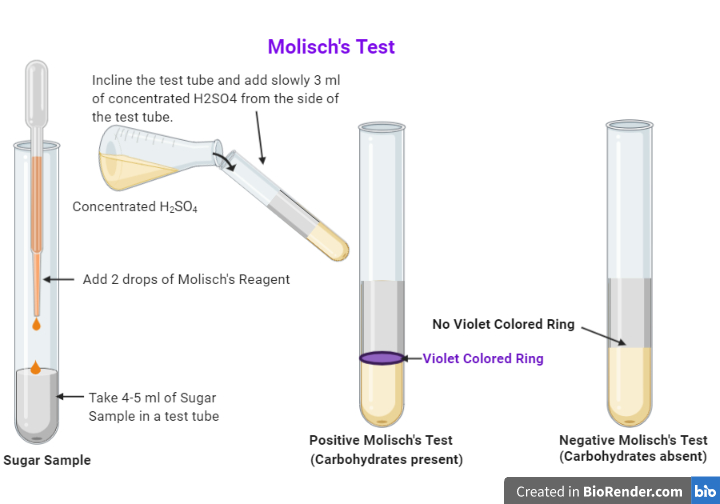
Molisch's Test Procedure. 2-3 drops of Molisch's reagent must be added to a small amount of the analyte in a test tube and mixed well. Now, a few drops of concentrated sulphuric acid must be added drop-wise along the walls of the test tube to facilitate the formation of a layer and avoid mixing. The development of a purple ring at the layer.

Molisch Testa group test for Carbohydrates YouTube
Limitations of Molisch Test. Trioses and tetroses do not have the necessary five carbon atoms for furfural formation, so they do not give a positive result for this reaction. Molisch test is not a specific test for carbohydrates. Furfurals as such or furfural yielding substance, some organic acids like citric acids, lactic acid, oxalic acid.

Molisch's Test Definition, Reaction, Reagent, and Procedure
The Molisch test is a highly sensitive colorimetric method used to detect the presence of carbohydrates, whether they are free or bound to proteins or lipids. It involves using a solution of α-naphthol (C 10 H 8 OH) in 95% ethanol (C 2 H 5 OH), which is known as the Molisch reagent . The formation of a purple or purplish-red ring at the site.

Molisch, Hans Biographien im AustriaForum
Molisch's test: reactives, sulphuric acid and 2% -naphtol in ethanol (a) and steps of procedure of application (b and c) Comparison of the results obtained between Molisch's Test and ATR- FTIR on.

Hans Molisch Alchetron, The Free Social Encyclopedia
Molisch's Test is a general test for all carbohydrates.

Hans Molisch (18561937), rector 1926/27 650 plus
Prepare a solution or suspension of the sample by placing ~0.1 g in 10 ml of water. Two ml of the test solution is placed in a test tube. Two drops of the Molisch reagent (a solution of -napthol in 95% ethanol) is added. The solution is then poured slowly into a tube containing two ml of concentrated sulfuric acid so that two layers form.

Molisch’s Test (Basic universal Test for Carbohydrates) YouTube
Molisch test is a colourimetric method for the analysis of the presence of carbohydrates in a given analyte. This test is named after Austrian botanist Hans Molisch. Molisch's test is done by using Molisch reagent. A solution of naphthol in ethanol (95%) is known as Molisch reagent. It's also known as the purple ring test.

Molisch’s Test Objectives, Principle, Reagents, Procedure and Result Online Biology Notes
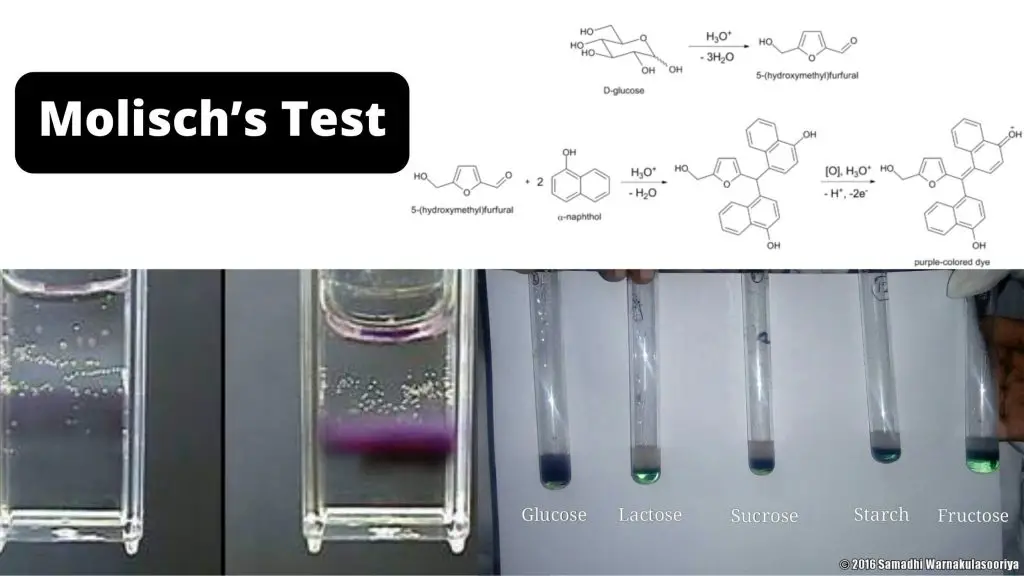
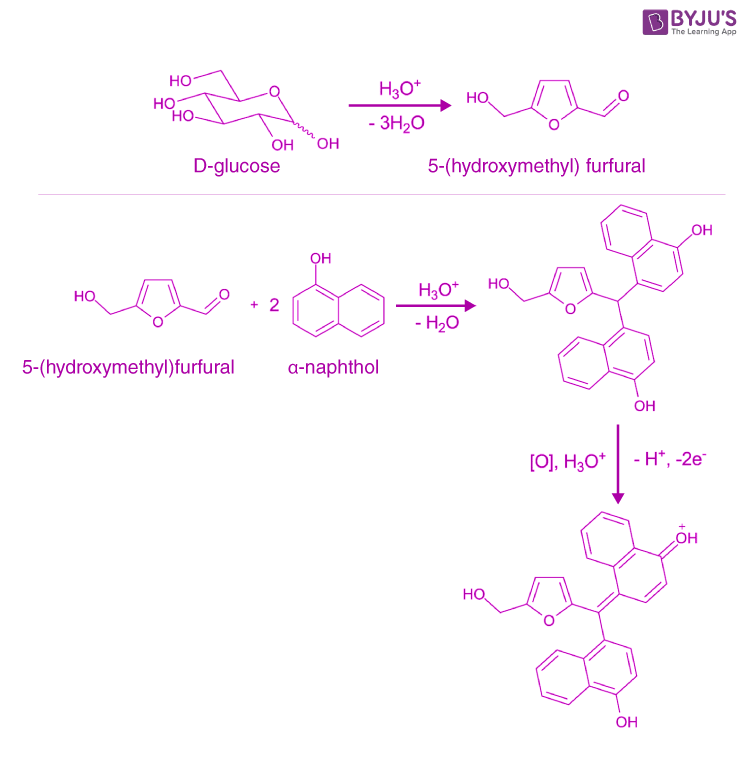
Molisch's test is a general test for all carbohydrates. In this test, carbohydrates when reacted with conc. H2SO4 get dehydrated to form furfural and its derivatives. When monosaccharide are treated with conc H2SO4 or conc HCl, -OH group of sugar are removed in the form of water and furfural is formed from pentose sugar and hydroxymethyl.
Molisch’s Test Objective, Principle, Procedure, Result
Molisch's test is a qualitative test used to detect the presence of aldehydes and ketones in a sample. The test uses a reagent made up of Schiff's reagent and concentrated sulfuric acid. When the reagent is added to a sample containing aldehydes or ketones, the aldehydes or ketones will react with the Schiff's reagent to form a colored.

PPT Lecture 1. WET METHODS OF CARBOHYDRATE ANALYSES PowerPoint Presentation ID323603
While Molisch's Test is widely used, it has certain limitations and interferences that must be considered. Factors such as pH, temperature, and interfering substances can affect the test results. For instance, substances containing phenolic groups can interfere with the reaction, leading to false-positive results.

Molisch's Test for Carbohydrates 2.0 YouTube
Molisch's test works on a simple principle which is explained elaborately in the next heading. Principle of Molisch's Test: To detect the presence of carbohydrates, the solution is first treated with a strong acid.This is for hydrolyzing the carbohydrate to monosaccharide. A compound named furfurol is then made when water is removed from.

Molisch's test Wikipedia
Book Abstract: "Professor Andreas F. Molisch, renowned researcher and educator, has put together a comprehensive, clear, and authoritative book on wireless communications. The Second Edition, which includes a wealth of new material on important emerging topics, ensures the book will continue to be a key resource for every student, researcher, and practitioner in the field."
Molisch’s Test Principle, Procedure, Reaction, & Reagent Preparation
Molisch test (using α-napthol) indicating a positive result (see purple ring). Molisch's test is a sensitive chemical test, named after Austrian botanist Hans Molisch, for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid or hydrochloric acid to produce an aldehyde, which condenses with two molecules of a phenol (usually α-naphthol, though other.
Molisch's Test Definition, Principle, Procedure, Result, Uses
Two ml of a sample solution is placed in a test tube. Two drops of the Molisch reagent (a solution of -napthol in 95% ethanol) is added. The solution is then poured slowly into a tube containing two ml of concentrated sulfuric acid so that two layers form. A positive test is indicated by:

Molisch's Test Practical Experiment YouTube
Molisch test is a chemical test to detect carbohydrates (monosaccharide, disaccharide, and polysaccharide) and glycoprotein in an analyte. It can be used to differentiate proteins and amino acids from carbohydrates. The test has been named after Czech-Austrian botanist Hans Molisch [1,2].